Google now indexes comments from Facebook and Google+ in search query results. Those comments are one reason that pharmaceutical brands have been slow to implement social
media. For good reason.
While marketers typically leave social on the table, the companies' IT departments are not shy about exploring emerging technology like radio frequency identification
(RFID) in an attempt to stop counterfeit drugs from entering the supply chain.
Police in China seized more than 65 million fake pills and arrested 114
suspects during a four-month investigation into counterfeit drugs, according to Associated Press. This type of counterfeit activity is more common worldwide than many want to believe. In an attempt to
protect consumers, Google has sued several advertisers for violating the search engine's policies
on the advertisement of illegal and fake pharmaceutical products.
advertisement
advertisement
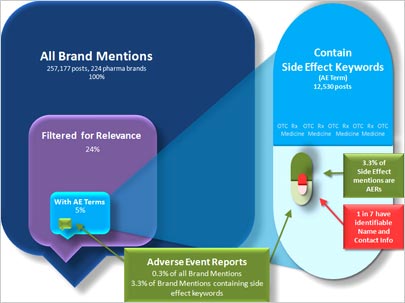
This type of publicity makes pharmaceutical brands guarded about social media. Testing the word of consumers, Visible
Technologies tracked 224 pharmaceutical brands, and collected and analyzed more than 257,000 posts across social media sites. The test, which analyzed the Adverse Event Reporting System (AERS) in
social media, ran for 30 days. AERS is a federal mandate requiring pharmaceutical companies to report certain adverse changes in health or side effects of their products discussed by users on social
media sites.
The purpose of the study was to understand how much of the conversation about
pharmaceutical brands on social media sites -- including forums, blogs and Twitter -- meets the requirements of AERS. It turns out the findings show that 0.3% of all posts contained an adverse event,
and even fewer met the federal reporting requirements, which are posts that provide contact details. Only 14% of posts that contained an adverse event had an identifiable name and contact
information to enable pharmaceutical marketers to fill out the required paperwork.
Jackie Kmetz, director of community education at Visible Technologies, said marketers have been hesitant to
use social media because of reporting requirements. Company marketers realize the benefits from online marketing, but there's a "scary big black hole" that obligates pharma marketers to fill out
paperwork and report events after a consumer makes an identifiable adverse event comment in social media, which includes name, content information and the specific name of drugs.
While people
aren’t talking about adverse events, it doesn't mean they don't happen. Google also started indexing Facebook comments in its search engine results earlier this year. Social media can be used
to help drugmakers identify potential safety issues with the product earlier in its lifecycle, minimizing the risk for a crisis and more serious patient outcomes. Social media also offers the
opportunity to foster customer relationships and establish more personalized healthcare services.
On average, each brand being monitored received a total of three posts that met the
requirements for AERS. So marketers can expect to see some mention of adverse events, but a low percentage of overall conversation happening online.