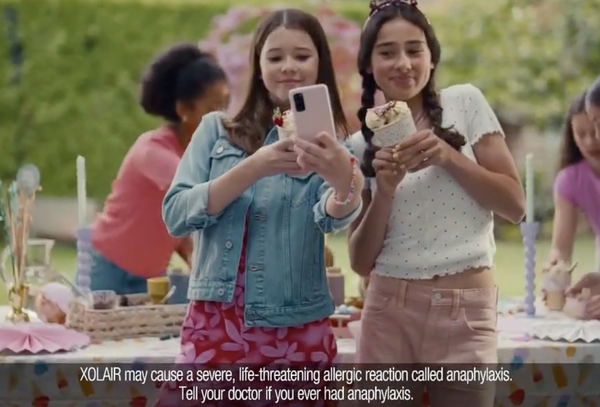
Image above: Old ads provide
side effects info against slice-of-life vignettes, a distracting no-no according to new rules.
Fox News used to call itself “fair and balanced,” whether or not the network
actually was.
Pharma commercials, meanwhile, have long been tasked with being “fair and balanced” in presenting a drug’s side effects and risks. And, under new rules in
effect since May 20, the Food and Drug Administration has also required that statement be “clear, conspicuous and neutral,” avoiding “audio or visual
elements…likely to interfere with comprehension of the major statement.”
The rules will start being enforced Nov. 20. Pharma & Health Insider recently discussed their
implication with Alexa Marshall, a senior vice president of creative excellence at market research company Ipsos, which helps nine of the top 10 U.S. pharma advertisers test ad concepts,
according to Marshall.
advertisement
advertisement
This Q&A has been edited for space and clarity.
Pharma & Health Insider: Will pharma companies really be compliant by Nov. 20?
Alexa Marshall: They're supposed to be. They have to present the drug information in a clear and really unobstructed way. We've seen a couple of clients start to dabble in that -- because we
pre-test ahead of when they're actually fully producing and airing the ads. But some have been a little bit slower.
P&HI: What have pharma spots been like up to now?
Marshall: You introduce the brand, then in comes the fair/balance, typically being shown when there's music played or when there are visuals happening -- when people are walking their dogs
down by the river, or other visual and audio elements.
That kind of slice-of-life, or vignette-style ad, has been almost 80% of the ads we have tested at Ipsos, because they've had to weave
the fair/balance in. With these new regulations, they really can't do that.
P&HI: What should happen instead?
Marshall: The information about potential side
effects is no longer allowed to be shown with anything that is going to distract the viewer from understanding that information.
The rule says you really can't have music in the background.
You can't have anything visual that is going to distract someone.
That leads to an opportunity, because right now they're sort of hamstrung. This new regulation allows them to take that [the
fair/balance statement] almost as a separate piece of a creative spot.
We're still seeing the beginnings of those potential opportunities -- how agencies will go about creating and how they're
going to do this.
P&HI: So what might a compliant commercial look like?
Marshall: You could have an ad where you have a full story about your product, your brand. One
scenario would be: the ad plays out and, at the end, you have a screen that truly reads through because they have to say audibly what the side effects are and show it visually at the same time without
anything else there.
This opens opportunities for brands to be much more creative, to help get more affinity for a specific brand.
P&HI: Will that help cut down on the
slice-of-life vignettes that have become so common?
Marshall: Exactly. It gives a little bit more freedom.
Pharma ads actually do break through to their intended audience.
Generally speaking, [when] a brand is advertising a specific product for a specific indication to someone who is a sufferer, that person tends to pay attention.
Where they [tend to fall short
relative to all ads and other categories is being able to really link to the brand. One of our tags – “[the] creativity fits with the way I feel about the brand” -- is really low
amongst pharma ads.”
This [the new rules] gives brands a real opportunity to work on creatively driving stronger brand affinity, closeness, and memorability versus just “I remember
seeing that ad, but it kind of looked like every other ad, and I don't really remember the brand.”
P&HI: Can you give an example of the 20% of pharma ads that currently
don’t use slice-of-life vignettes?
Marshall: They’re more unbranded disease state awareness ads: the advertiser is just trying to get people aware of something, and
[there’s] a call-to-action to talk to your doctor to find out more. Those tend not to be slice-of-life, because they're not beholden to fair/balance, and not selling a specific product.
P&HI: You said earlier that you've seen a few ads that are already being more creative because of the new rules. Can you provide any examples?
Marshall: The ones we
have seen are the opposite of slice-of-life. They're trying different creative techniques. They might have sort of testimonial-style [or] they're using different celebrities.
P&HI: With just weeks to go until enforcement of the new rules, what advice would you give to a pharma company at this point?
Marshall: Our advice would be test different
things in a safe space, push the limit, try different types of creative tactics, ways you haven't tried before that you now have the leeway to do.
Entertainment is not a phrase you typically
use with pharma advertising.
They tend to do well in terms of being informative, telling you something new when you’re selling a product that I need. But they really can do better
by enhancing those creative experiences more so than what pharma ads have done in the past.