Photo Credit: Tanya
Gazdik/MediaPost
Depending on which state consumers live in, COVID booster vaccines may or may not be available or may require a doctor's prescription.
“CVS and
Walgreens, the country’s two largest pharmacy chains, are for now clamping down on offering Covid vaccines in more than a dozen states, even to people who meet newly restricted
criteria from the Food and Drug Administration,” according to The New York Times.
“Walgreens said in a statement that it was ‘prepared to offer the vaccine in states where we are able to do so’ to people who met the F.D.A. criteria. When a New York Times reporter
tried to schedule vaccine appointments in all 50 states, the Walgreens website said patients would need a prescription in 16 of them. Though there is some overlap, it’s not the same set of 16 as
CVS, underscoring the level of confusion.”
advertisement
advertisement
COVID vaccines had been available to anyone 6 months and older regardless of their health.
“That's no
longer the case,” according to NPR. “Now, the FDA is limiting the updated shots to those who are
at risk for serious complications because they are 65 or older, or have other health problems. These new changes may bring back questions that are reminiscent of the early days of the
pandemic.”
COVID vaccines are no longer available at CVS stores in Massachusetts, Nevada and New Mexico and available only with an authorized prescriber's
prescription in 13 states and the District of Columbia, according to Axios.
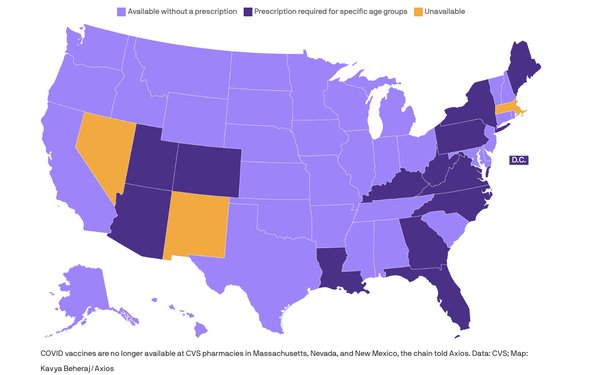
Photo Credit: Axios
Robert F. Kennedy Jr., secretary of the U.S.
Department of Health and Human Services, recently fired the director of the U.S. Centers for Disease Control and Prevention, triggering the resignations of several top leaders.
“Amid unprecedented and sweeping changes to federal vaccine policy, Dr. Natasha Bagdasarian, Michigan's chief medical executive, said public health officials in states like
Michigan are taking the lead in determining how COVID-19 vaccines will roll out in their communities this fall,” according to the Detroit Free Press. “Michigan and other
states are ‘now taking a stronger leadership role’ and are issuing their own recommendations to align with the American Academy of Pediatrics and the American College of Obstetrics and
Gynecology.”
When the U.S. Food and Drug Administration recently approved labels for the vaccines, it limited the Pfizer COVID-19 vaccine, authorizing it only for use in
adults ages 65 and older and for people ages 5 to 64 with at least one health condition that puts them at high risk for severe disease from the virus.
“Most of these
conditions can affect the immune system in such a significant way that they would inhibit the body’s ability to fight off a Covid-19 infection, increasing the risk of that infection causing
severe illness or death,” according to CNN Health. “These underlying conditions
will make an estimated 100 million to 200 million people in the US eligible for a Covid-19 vaccine under the new framework.