In 2020, at the height
of the pandemic, I began paying regular visits to a speech therapist for the treatment of chronic cough.
“Have you been coughing during the past few days?” the Mr. Sinai
receptionist would ask masked me as part of her COVID routine. “Well, yes,” I would answer, “that’s why I’m here!”
Then, wearing one of those see-through
plastic headpieces, I would meet with my therapist for weekly exercises in behavioral cough suppression therapy (BCST).
If only there had been an easier way.
Turns out that, at the
same time, a startup called Hyfe was introducing a free app, now called CoughPro, to monitor all the coughing then going on, from whatever cause. To date, more than 100,000 people have used the
app.
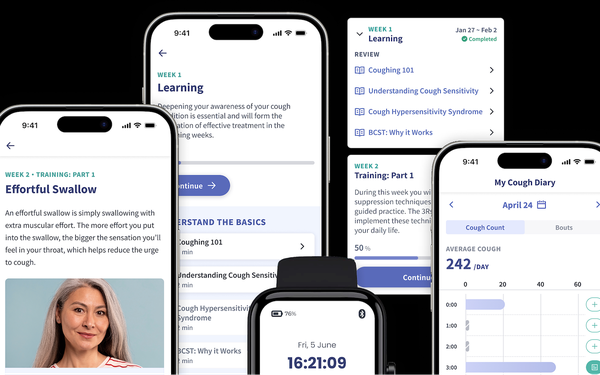
But consumer cough monitoring is not Hyfe’s reason for being. Instead, the company aims to combine the app’s capabilities with digital therapeutics, offering BCST as a
telehealth service.
advertisement
advertisement
That’s why, from 2022 to early 2024, Merck was promoting CoughPro as part of its ambition to bring Gefapixant to market as the first new cough drug in the US since
1958.
Gefapixant didn’t survive the FDA approval process in the USA, but Merck is selling the drug in Japan through Kyorin Pharma -- which also just partnered with Hyfe to bring the
latter’s combo of CoughPro and behavioral cough therapy to market as a medical device. Kyorin and Hyfe plan to clinically test the pipeline product in 2026 under the Japanese regulatory
process.
Hyfe, meanwhile, is also seeking partners -- whether pharma companies, OTC companies, or health systems -- to launch the product in the U.S. and elsewhere.
I spoke with Hyfe's
CXO (chief experience officer) Reid Moorsmith on March 12, the fifth anniversary of the company’s launch.
This Q&A has been edited for length and clarity.
Pharma & Health Insider:How does behavioral cough suppression therapy work?
Reid Moorsmith: It is a very well-evidenced behavioral therapy combing education, cough
suppression techniques and coaching that enables people who have refractory chronic cough to treat it without drugs.
Unfortunately, it’s only offered by about 200 speech language
pathologists. Our team has Dr. Laurie Slovarp, who’s one of the world’s leading experts in BCST. We work to put that therapy in the form of an app to make it widely accessible.
P&HI:What research backs up your product?
Moorsmith: Over 50 trials at this point have used the Hyfe technology. And BCST itself has quite a lot of research behind
it. One study showed a 41% reduction in coughs, another showed that over 80% of the participants said they were satisfied or very satisfied with their treatment.
P&HI: How does your
recently announced partnership with Actigraph fit into research studies?
Moorsmith: Actigraph is a leader in wearables in the clinical trial world, where sponsors want to measure
numerous endpoints, and cough is one of them.
P&HI: Is Hyfe looking at wearables for its own digital therapy?
Moorsmith: We have a prototype, the “cough
watch,” that uses an Android device already on the market for detection and counting of coughs. We’ve demonstrated that it works in that form, and also want to ensure that it can
work alongside the other features of smartwatches.
It’s important the device can run for as long as it needs without needing to be recharged. We expect in the near future to be able to
partner with major consumer tech wearable companies to embed cough monitoring within what they already offer.
P&HI: What kinds of consumer outreach have you done for CoughPro?
Moorsmith: When we were in partnership with Merck, they did quite a lot of advertising, and that increased the number or users of our app substantially. Independently, we’ve done very
little marketing. We’re not trying to be an app with millions of users like Calm or Headspace or Noom. That’s not our primary business model. We use a little bit of advertising just to
make sure there are people using our app, and we learn from those people so that we can improve our offerings in partnership with others.
P&HI: How large is the potential
market?
Moorsmith: Most estimates say that between 5% and 10% of adults have chronic cough. Some have chronic respiratory disease like COPD or asthma, but some have refractory or
unexplained chronic cough with no underlying disease, where nothing is causing the cough other than the neural pathway that leads people to feel the sensation that they have to cough -- and then to
cough in response to that sensation. That loop, called cough hypersensitivity syndrome, is quite common among people who have chronic cough, and that’s the condition the digital therapeutic will
treat.