Hot topic: Oh, Oh, Oh Ozempic -- and other
prescription meds like Mounjaro and Wegovy initially developed for type 2 diabetes, but now widely prescribed for weight loss.
Not only has their surging use against obesity led to sporadic
shortages adversely affecting diabetes sufferers, but last week the pharma companies behind them got a double jolt:
1. A lawsuit alleging lack of warning about possible severe stomach
disorders that was filed by a Louisiana woman who was first prescribed Ozempic and then Mounjaro.
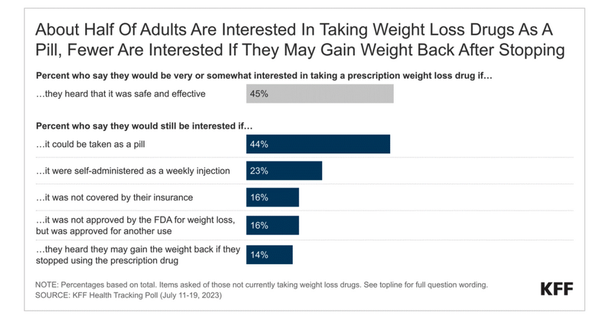
2. A KFF Health Tracking Poll showing that, despite the drugs’ popularity, Americans are
averse to their high costs, the need to take them by injection rather than orally, the fact that some have not been approved for weight loss, and the tendency to gain weight back after stopping
treatment.
In the poll, nearly half of all U.S. adults (45%) expressed interest in taking a “safe and effective” prescription weight-loss drug,. This included 59% of those
currently trying to lose weight and 51% of those trying to lose less than 10 pounds.
advertisement
advertisement
Seven in 10 respondents said they had heard at least “a little” about Ozempic, Wegovy or
Mounjaro.
Bu, as KFF points out, only Novo Nordisk’s Wegovy has been approved for weight loss by the Food and Drug Administration (FDA). The other two have yet to be deemed “safe
and effective” by the FDA – Eli Lilly’s Mounjaro is awaiting such approval, while Novo Nordisk has yet to submit a new oral pill weight-loss version of Ozempic to the FDA.
Indeed, the fact that all three medications currently must be taken by injection throws a wrench into their potential use. Only 23% of all adults said they would be interested in taking weight-loss
medication by injection.
And, working against Mounjaro and Ozempic for the moment, only 16% would be interested in taking a medication that the FDA has not approved specifically for weight
loss,
Other roadblocks include lack of coverage by insurance (only 16% would still be interested) and the possibility of gaining weight back after stopping use (14%).
As to the latter,
Novo Nordisk earlier this year said that people could be back to their original body weight five years after stopping Regovy, and regain half of their weight lost in two to three years.
On the
plus side for Novo Nordisk and Lilly, 75% of those polled said they trust pharmaceutical companies to develop new drugs, 66% trust companies to provide reliable information about safety and side
effects, and 64% trust pharma firms on drug effectiveness.
On the negative side, though, only 22% trust pharma companies to price products fairly, with 83% -- including majorities of both
Democrats and Republicans – feeling that drug company profits are a major factor contributing to the cost of prescription drugs.
More regulation of drug prices is favored by members of
both parties, but a majority of people – including those aged 65 or older – are unaware that the year-old Inflation Reduction Act contains provisions to reduce the price of drugs under
Medicare. And just 5% of people know that pharma companies will be penalized for increasing prices faster than inflation for Medicare recipients.
The KFF Health Tracking Pollwas
conducted July 11-19, online and by telephone among 1,327 adults